CANDIDATE FOR COVID-19 VACCINE AND ITS PATENT RIGHTS
At the end of the 9th month since the COVID-19 virus infected Indonesia and there are even other countries that were first infected by the virus, the time has come for each country to compete to end this pandemic by means of vaccinations. When the social distancing and lockdown roads are felt to be no longer effective due to people who are tired of implementing them and the demands of the wheels of life that must continue to run, what people are waiting for the most is the presence of a vaccine to restore their former productive life rhythm.
Community fatigue in implementing social distancing and lockdowns certainly has an impact on the increasing number of positive cases every day. Seeing this fact, there have been several countries holding trials for the Covid-19 vaccine, even several pharmaceutical companies have prepared to produce it and apply for a patent for the Covid-19 vaccine before it is distributed to the public.
It was recently discovered that the Chinese authorities issued the first patent rights for a vaccine candidate for the Corona COVID-19 Virus, Ad5-nCOV. The patent was granted to Chinese vaccine specialist CanSino Biologics Inc. The state newspaper People’s Daily quoted a document released by China’s National Intellectual Property Agency, which stated that the patent rights for the COVID-19 vaccine made by CanSino were issued on August 1.
So far there are three vaccine candidates developed by China. The three of them are now entering phase 3 trials. The three vaccine candidates from China are Sinovac, Sinopharm, and CanSino.
- a) The Sinovac vaccine, is currently being tested in a number of countries, including Indonesia and Bangladesh. In Indonesia, Padjadjaran University took part in the trial which involved 1,620 volunteers. The Corona Virus vaccine will be injected twice into volunteers per 14 days. Periodically, the team will conduct supervision and inspection of each volunteer. Volunteer monitoring was carried out for 7 months.
- b) Sinopharm vaccine, tested in the United Arab Emirates. Health authorities issued permits to find 15 thousand volunteers for the Corona COVID-19 vaccine test. The UAE Ministry of Health is looking for volunteers aged 18-60 years who have never previously contracted the Corona COVID-19. People with cancer or immune deficiency cannot participate.
- c) CanSino Vaccine conducts Phase III clinical trials of the Covid-19 vaccine candidate, Ad5-Ncov, in Saudi Arabia. This vaccine consists of a harmless flu virus known as adenovirus type 5 (Ad5) to transmit material from the corona virus into the body.
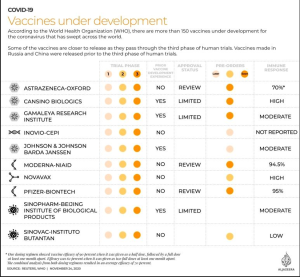
Regarding the Sinovac vaccine, news circulated that the COVID-19 vaccine developed by Sinovac Biotech was the weakest when compared to other candidates. This news came after in November, Al Jazeera launched an article containing a table on the development of clinical trials of various COVID-19 vaccines.
The table in detail compares 10 COVID-19 vaccines, including from AstraZeneca-Oxford, CanSino, Gamaleya Research Institute, INOVIO, Johnson & Johnson, Moderna, Pfizer-BioNTech, Sinopharm, and Sinovac. It is said that the COVID-19 vaccine that causes the highest immune response is from Pfizer with a rate reaching 95 percent. Then the weakest is the Sinovac without numbers, only the description “low”.
It is different from China, in contrast to the United States, which is said to have signed a contract worth US $ 1.5 billion (around Rp.22 trillion) with drug producer Moderna Inc regarding the purchase of 100 million doses of its COVID-19 vaccine candidate. One dose of Moderna costs around $ 30.50 A (around Rp. 449,197) per person for a two-dose package.
Referring to the many potential COVID-19 vaccines that are currently being developed and some are known to have very good and very low capabilities, what is expected now is that the vaccine with the best quality and performance can be provided and used not only by superpower and other developed countries, but can also be given to all countries including Indonesia. Putting aside the commercial value of the vaccine above human value so that the true victory against the COVID-19 virus will be ours, not just certain countries.
Sources:
https://health.detik.com/berita-detikhealth/d-5302737/benarkah-vaksin-covid-19-sinovac-paling-lemah
https://www.antaranews.com/berita/1663402/as-teken-kontrak-pembelian-vaksin-covid-19-moderna-senilai-rp22-t